How to successfully transition to an Electronic Lab Notebook
![]() 5 min read
5 min read
Case study by NEUWAY Pharma, Germany, focused on preclinical and clinical development of innovative therapeutics.
By Dr. Jonas Kosten
Division Leader Analytics
NEUWAY Pharma
Germany

About NEUWAY® Pharma
NEUWAY focuses on the pre-clinical development of innovative therapeutics for treatment of severe diseases with high medical need based on its proprietary Drug Delivery Platform so-called EnPC® (engineered protein capsules), particularly useful for the treatment of brain diseases.
EnPC® forms a virus-free protein capsid having a natural built-in on target property for delivering drugs. The capsids enables the encapsulation of different drug modalities (nucleic acids, antibodies, small molecules) that do not cross the physiological blood brain barrier (BBB). Combining EnPC® technology with a nucleic acid-based transcript therapy represents a unique approach for gene therapy.
“Main benefit and reason for having an electronic lab notebook is the creation of a searchable database, accompanied by significant time savings.” – Jonas Kosten, PhD, NEUWAY Pharma
See the presentation with practical and strategic advice:
Take away messages
ELN selection process framework to identify and implement an ELN can be divided in 4 main steps:

1 – Analysis of Lab workflow and lab needs
At NEUWAY Pharma there are three major Team categories:
- Standard Production: defined/fixed processes & analytic methods, SOPs, defined dataset; standardized documentation format; transfer of material and QC data to other teams
- R&D: variable protocols, different methods & data; collaborative
- Analytics: set of analytical methods, SOPs; part of different experiments (service-like); instrument databases
Important things to address when defining the needs of the lab:
- Know your Processes (within & between teams). Talk to your colleagues.
- Format & requirements of Protocols (e.g. SOPs)
- Understand your Data generation (step, format)
- Documentation: What goes into lab book & what doesn‘t (digital)
- Review: Signature process
- Data analysis
- Existing digital systems in your lab/ company? (e.g. instrument databases)
2 – Market research – Develop your understanding of ELNs
- What are the costs of an ELN?
- What are the standard features of ELNs? Where do they differ?
- What features are essential, which ones are nice to have?
- How can you transfer and map your processes in the ELN?
- Iterate between Status Quo Analysis & Market research
Organize and include your team from the start:
- Contact vendors to organize demos
- Include as many users as possible in the demo sessions and get their feedback
- Helps in ELN pre-selection
- Serves as introduction to ELNs for team
- Expectation management
- Identify members for your testing team
3 – Electronic lab notebook selection – How to evaluate pricing, service and make a choice
Start with the short-list generation:
- Make a list of criteria to narrow down search and focus on your must haves (e.g. price below 10.000€/year/10users)
- Use Must have criteria to select some ELNs for in depth testing
- Assemble your testing team. Make sure the testing team members come from different teams, enjoy working digital and have good time management skills.
- Initiate test trial
ELN vendors prioritization and testing:
Criteria Categories (examples)
- Costs (including setup & hardware)
- IT (e.g., cloud vs. on-premise, security features, backups, migration, maintenance effort)
- Regulatory compliance (e.g., electronic signatures, audit trail, validation)
- ELN features (e.g., office integration, search function, templates, supported data formats)
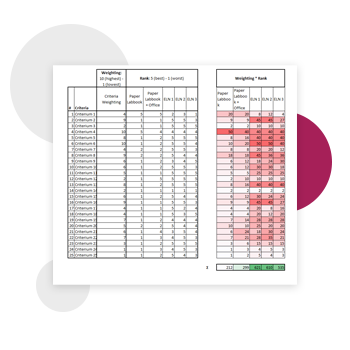
Rate the criteria according to importance. It is important to involve decision makers, and find consensus. Then, compare ELNs using your criteria (who’s best in most important criteria, according to your decision matrix). Also, include the status quo in the comparison (e.g. paper lab book + office).
Here is how NEUWAY Pharma set up an Excel spreadsheet to help them evaluate various ELN options:

Image legend & explanation of criteria: 1) lock of data/document 2) Internal Links/Cross-references (Documents, SOPs, Samples…) 3) (e.g., filtering, tracking) 4) Easy transition from current system – Effort & Transfer 5) Easy import of tables\diagrams\picture\… 6) Export and Print of documents (sharing) 7) right management (read/write) 8) (samples, ordering, barcodes, tracking) 9) Organization can be mapped onto ELN structure 10) Weighting: 10 (highest) – 1 (lowest)
Get the NEUWAY Pharma’s template
Download the Excel template by NEUWAY Pharma for your ELN evaluation process.
4 – ELN implementation – How to encourage the lab team to implement and use the ELN?