Ensuring Research Continuity: 5 Keys to Lab Inventory Management During Transitions
We often view lab inventory management as simply the organization and tracking of materials within laboratory settings. However, trouble comes when there is a transition – either staff leaving, moving to a new location, or accidents – and critical inventory information is lost forever.
In this blog post, we will highlight 5 crucial inventory management strategies that can directly prevent the loss of your vital experimental information, ensuring your research endeavors remain on track.
We also discussed these strategies within the webinar “Staying Ahead of the Shuffle: 5 Keys to Lab Inventory Tracking in Ever-Changing Environments.” You can watch the webinar below.
Key Summaries:
- Rethinking lab inventory management: Typical views of lab inventory miss its vital data aspect, which can get lost during transitions. Recognizing lab inventory management as data management is crucial for research continuity.
- FAIR principles in lab inventory management: Integrating inventory data with lab data strategies ensures it’s Findable, Accessible, Interoperable, and Reusable, promoting collaboration and efficient research practices.
- Five essential strategies to prevent inventory data loss: Lab leaders need to digitize inventory management, detail protocols, conduct regular audits, automate processes, and prioritize robust backups to protect critical inventory data and optimize operations.
Lab Inventory Management: More than tracking boxes and bottles
We typically treat inventory management only about having systematic control and organization of materials used in laboratory settings. It involves tracking the location, quantity, and distribution of various items essential for experiments and research.
Lab inventory management will answer questions such as: Where is item A stored? How much item B do I have left? Where can I order item C from? Or do I have enough item D for experiments?
However, in asking these questions, some additional questions and information would arise:
- Where is item A stored? What instructions come with using item A? Where are MSDS/data sheets/QA docs filed? What conditions has item A been stored under?
- How much item B do I have left? Which experiments used item B? Who conducted the experiments? Which lot/subtype of item B was used? How was the protocol using item B developed? (Vendor? Collaborator? Publication?)
- Where can I order item C from? What accounting information is associated with ordering item C?
- Do I have enough item D for experiments? How was item D prepared? What do I need to prepare more?
These could be considered data and metadata associated with your inventory management. The management of such data can perhaps be called “Inventory Data Management.”
Inventory, data, and the FAIR principles
Answers to these questions are typically lost during transition – either when moving your lab to another location, having staff and student turnover, encountering accidents, or being away from your lab for an extended period (like during 2020…). This is not surprising, because the same thing happens with your lab data and metadata in situations like these. Missing inventory information can lead to scenarios such as:
- You are asked to reproduce the results of an experiment, but you have no idea which specific sample or reagent was used in an experiment done by a lab member who left 2 years ago.
- You are not sure who developed the protocol to extract a particular sample or to generate a specific data set, or how the protocol was developed.
- What associated MSDS/data sheets/QA docs are filed and where.
- And more.
To tackle these issues, we must think of inventory management as data management, and incorporate inventory “data management” into your lab data management strategy. This means, we should consider the FAIR principles to make sure inventory data is findable, accessible, interoperable, and reusable.
From the inventory data perspectives, this means:
- Inventory metadata & data should be easy to find for both humans & computers.
- Users know how inventory data can be accessed (plus authentication & authorisation)
- Inventory data should be integrated with other data, and interoperate with applications or workflows (for analysis, storage, and processing).
- Inventory metadata and data should be well-described so that they can be reused – replicated and/or combined in different settings.
To achieve these, there are 5 keys that every lab leader and manager should follow to avoid the loss of critical lab inventory information.
1. Seriously, keep inventory management digital!
We can’t emphasize this enough – if inventory management and its data isn’t digital, and if it isn’t part of your existing data management structure (which should be digital, too), it is extremely difficult to connect inventory information to data.
Imagine digging through boxes of inventory invoices or staff notes just to find the catalog number of a particular reagent. Digitalization means you can embed this information directly into your digital lab notes through smart annotation, hyperlinks, or attachments. You can find what you need in a few clicks, or through a quick search in your notes.
Also, all those supplementary materials on paper? Photograph or scan them, turn them into digital formats, and file or attach them digitally.
Here is a poll we ran during the recent “Staying Ahead of the Shuffle: 5 Keys to Lab Inventory Tracking in Ever-Changing Environments” webinar. While most respondents have adopted digital tools for inventory management, 38.8% still rely on paper, either exclusively or as part of their tool set.
2. Details and specificities matter
Make sure to incorporate inventory information in protocols and lab notes, and be as specific as possible. In fact, to ensure traceability and simplify tracking, it is best to assign a unique ID to each inventory item and refer to each item by this ID, to avoid any confusion and ambiguity.
Some strategies to handle this include:
- Have a standard naming convention for inventory items and include unique ID assignment within the lab’s inventory management processes (e.g., assign the ID as soon as an inventory item is received and logged). This can be done manually, but some electronic lab notebooks (ELNs), like SciNote, would do this automatically.
- Use hyperlink or the ELN’s cross-referencing system (e.g., smart annotations in SciNote) to assign or refer to specific inventory item within experiment notes or have a clear path to refer to results of analysis. This means that when you revisit the lab notes again, you will know exactly which item was used, and have the item’s information readily available.
- Some ELNs have existing integrations with lab inventory management systems (e.g., Quartzy), which allows for cross-referencing inventory items within the ELN.
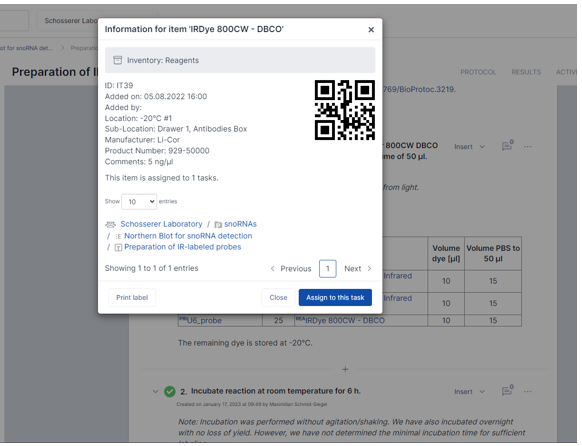
SciNote Electronic Lab Notebook comes with a built-in inventory management system. Each inventory item is given a unique ID automatically, and you can easily assign inventory items within experiments, or find the item’s information with one click. Image provided by Markus Schosserer.
3. Review lab inventory and lab notes regularly – and ensure you have full access
Here is another scenario to avoid: when a lab member leaves, you either can no longer find the notes, or can’t understand the notes due to messy handwriting.
With regular audits and reviews, you can ensure that you have access to inventory information and lab notes, and spot any irregularities in documentations (e.g., missing references to inventory items). Even better, develop some standard templates for lab data and inventory data documentation, so everyone records information in the same manner. Finally, if you are a manager, make sure you have full access to inventory information and lab notes.
Here are some other tips that might help:
- Incorporate reviews or audits into routine lab activities, such as lab meetings or quarterly business reviews. Or, schedule them to occur regularly.
- Take advantage of the data access controls provided by data management platforms, so you know you have access to data even after a user leaves.
4. Automate whatever you can – integrate and connect
There are many reasons to automate inventory management, especially if you deal with a high volume. It can help you save time, reduce manual entry errors, and maintain consistency. A lot of the digital tools, such as ELN notebooks, come with the ability to assign unique IDs, generate labels/barcodes, or to template processes. Some also offer APIs – application programming interfaces – that labs can use to integrate external inventory management systems, or with other data platforms such as SharePoint.
These will help keep the lab inventory processes (including data management) and traceability intact. Digital platforms that provide an activity log will also help trace what was previously done.
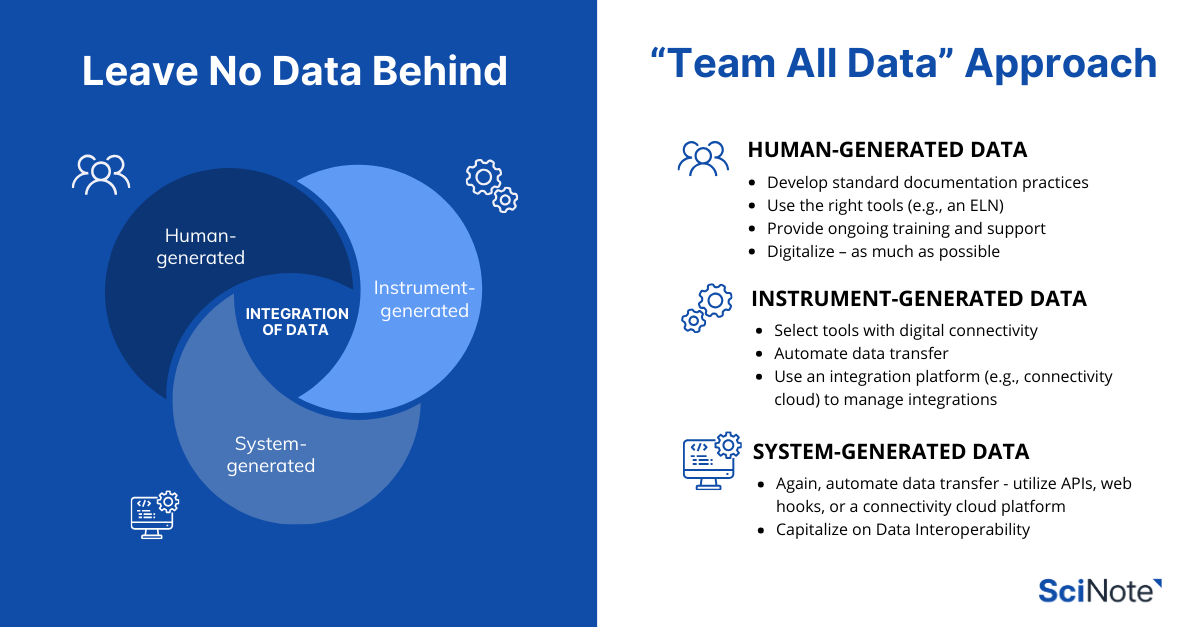
Do keep in mind that API implementation does require some technical understanding. Another option is to utilize a cloud connectivity platform to support data inputs from other sources, such as equipment and databases. The more data capturing can be connected and/or automated, the better – here, we suggest a “Team All Data” approach.
5. Backup, Backup, Backup
A spilled coffee, a misplaced USB drive – disasters can strike in unexpected ways. That’s why backing up everything is so important. Whether it’s safeguarding against natural disasters, navigating personnel changes, or mitigating human errors, ensure that backup mechanisms and archiving systems are in place.
When selecting a digital platform for storing your data and inventory information, look for those that back up regularly, and in different geographical locations, to ensure you can retrieve your data regardless of what happens.
Pro-tip: Have someone in charge of managing the inventory (and its data)
A common mistake is to think that maintaining data – including inventory data – doesn’t require resources. All the steps outlined before does require someone to own them. So, a recommendation from us is to ensure someone on your team is responsible for this.ha
Treat inventory management as data management
Effective lab inventory management goes beyond organizing bottles and tubes; it involves safeguarding inventory information vital for research continuity. By digitalizing inventory data, integrating inventory details into protocols and notes, implementing regular audits and reviews, automating operational processes, and instituting robust backup mechanisms, laboratories can ensure the preservation and accessibility of their inventory data, even amidst transitions.
These strategic measures will mitigate the risk of encountering scenarios that will result in the loss of your vital experimental information – and will foster an environment that will support research productivity and success.
What’s your next step for inventory (data) management?